Corrosion
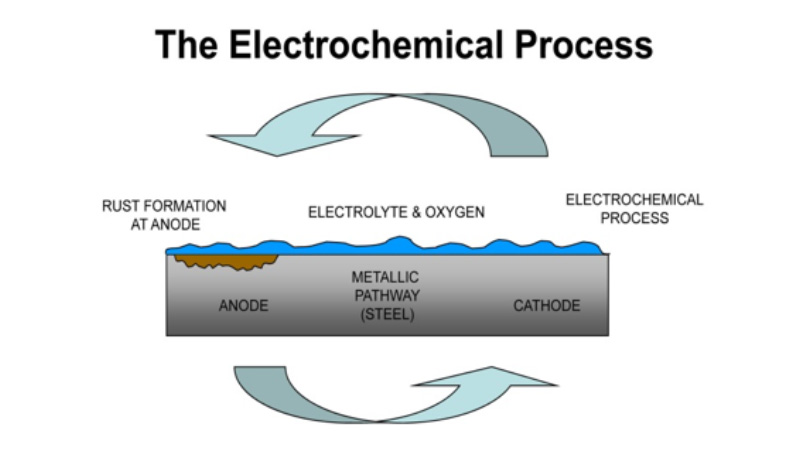
Corrosion has a huge economic impact due to its immense damage to infrastructure such as highways, bridges, power lines, etc. This requires extensive repair and damage control which has immense costs to governments, industries and the general population who are affected by the damage and repair processes. The annual cost to repair damage from corrosion adds up to around $1.8 trillion worldwide. Corrosion is a naturally occurring development which can be defined as the decay and deterioration of materials, in our case – metals such as steel, copper, iron, etc.